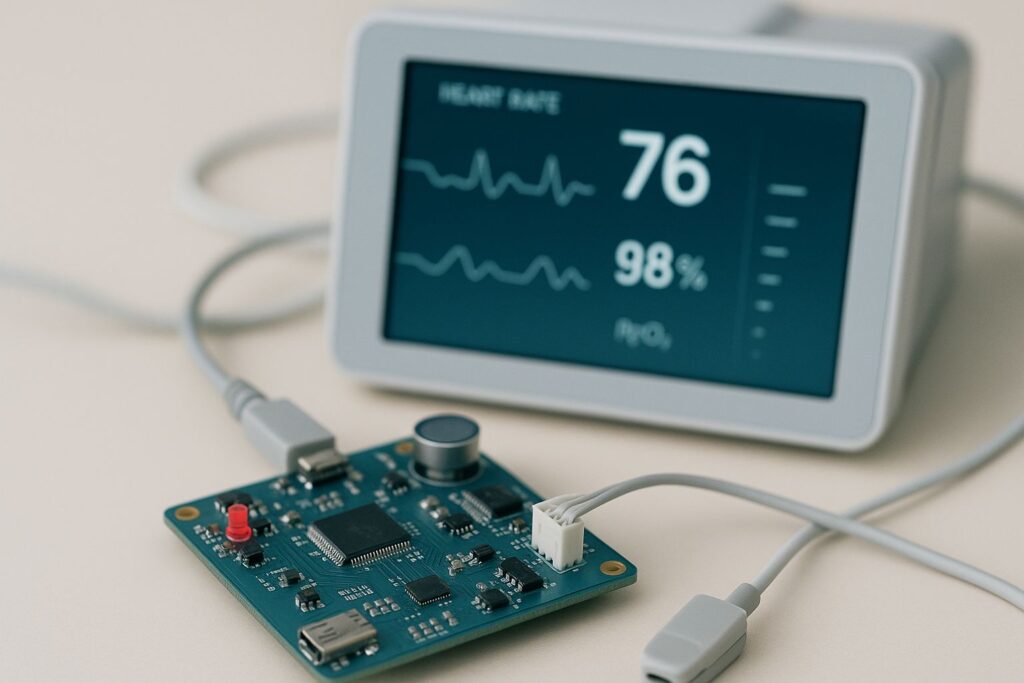
Hardware-software integration in medical devices involves ensuring that embedded software can effectively communicate with sensors, actuators, and other hardware components. Common challenges include protocol mismatches, timing issues, and hardware limitations. To resolve these, teams should use modular architecture, follow regulatory standards like ISO 13485 and IEC 62304, adopt agile collaboration methods, and conduct robust system-level testing before deployment.
🩺 Introduction
Brief Overview:
Modern medical devices have evolved into highly sophisticated systems that rely on a seamless integration of hardware and software. Hardware components such as sensors, actuators, and microcontrollers work hand-in-hand with software that processes data, manages control logic, and enables user interfaces.
Yet, achieving this integration is not simple. Even minor discrepancies—like mismatched communication protocols, timing issues, or hardware limitations—can cause major malfunctions, potentially risking patient safety and delaying regulatory approvals.
Importance and Relevance:
Hardware-software integration in medical devices is mission-critical. A flaw in integration doesn’t just lead to a technical error—it can directly impact a patient’s life, trust in medical technology, and the manufacturer’s reputation.
-
Regulatory authorities like the FDA, ISO, and IEC require comprehensive testing to verify safe and effective integration before a device reaches the market.
-
The growing demand for smart medical devices, remote health monitoring, and AI-driven diagnostics increases the complexity of integration.
-
Effective integration ensures faster product development, regulatory compliance, and improved patient outcomes, while reducing the risk of recalls or litigation.
What You’ll Learn in This Article:
In this article, you will discover:
-
What hardware-software integration means in the context of medical devices.
-
The most common integration challenges that developers and engineers face.
-
Proven best practices and design strategies to solve these issues.
-
Insights from real-world case studies, including success stories and failures.
-
Actionable guidance on how to manage teams, meet regulatory demands, and build more reliable devices.
🔹 Section 1: Understanding Hardware-Software Integration in Medical Devices
Key Points:
-
Define integration in the medical device context
-
Common integration challenges
-
Importance of cross-disciplinary collaboration
1. What is Hardware-Software Integration in Medical Devices?
In medical devices, hardware-software integration refers to the process of making sure that the electronic components (hardware) and the code (software) work together in harmony. This is essential for devices like insulin pumps, ECG monitors, pacemakers, and wearable health trackers.
Example:
In a digital blood pressure monitor, the sensor (hardware) measures pressure, and the software translates this signal into readable data on the screen. If integration fails, the device might display incorrect readings or crash entirely.
2. Common Integration Challenges
Despite careful planning, many development teams face significant integration hurdles:
-
Communication Mismatches: If the software expects I²C and the hardware is set up for SPI, or if there’s a mismatch in voltage levels, the device won’t function properly.
-
Synchronization Issues: Real-time devices like ventilators require perfect timing. Delays or jitter in software can cause unsafe hardware behavior.
-
Real-Time Performance Bottlenecks: Limited processing power or memory on the hardware can slow down software tasks, especially when advanced features like machine learning are added.
3. Why Cross-Disciplinary Collaboration is Vital
Integration can’t succeed in isolation. Hardware and software teams must work together from the earliest design stages:
-
Electrical Engineers design the circuitry and choose components.
-
Software Developers write firmware and applications.
-
System Architects ensure everything aligns within performance and safety standards.
Pro Tip:
Using shared tools (like Jira or GitHub) and regular alignment meetings can eliminate misunderstandings that cause integration failure later on.
In summary, understanding integration is the first step to resolving its challenges. It’s not just a technical process—it’s a team effort that combines precise engineering with smart planning.
🔹 Section 2: Identifying Common Integration Challenges
Key Points:
-
Interface mismatch or communication protocol conflicts
-
Timing and synchronization problems
-
Hardware limitations affecting software performance
1. Interface Mismatches & Communication Protocol Conflicts
One of the most frequent causes of hardware-software integration failure in medical devices is incompatible interfaces. These problems arise when the software and hardware aren’t speaking the same “language.”
-
Common Conflicts:
-
SPI vs. I²C vs. UART: If the hardware is set to communicate using SPI (Serial Peripheral Interface) but the software expects I²C (Inter-Integrated Circuit), data transmission will fail.
-
Voltage Level Issues: Some hardware components operate at 3.3V while others use 5V, leading to signal misreads or damage if not properly handled.
-
Real Example:
A digital thermometer failed to transmit data to its display module because the developer assumed a UART interface, but the chip only supported I²C—resulting in weeks of redesign.
2. Timing and Synchronization Problems
Medical devices often function in real time. Synchronization between software and hardware must be tight and predictable.
-
What Can Go Wrong:
-
Missed interrupts from sensors
-
Data lag due to poor buffering
-
Inconsistent sampling rates
-
Why It Matters:
In devices like infusion pumps or ECG monitors, a few milliseconds of delay can lead to incorrect dosing or missed heartbeats—both of which could be life-threatening.
3. Hardware Limitations That Bottleneck Software
Many embedded medical devices run on microcontrollers with limited RAM, ROM, and processing power. Software that isn’t optimized may:
-
Crash due to memory overuse
-
Run too slowly to be useful
-
Drain the battery faster than expected
Example:
A wearable glucose monitor had to abandon a complex data analytics feature because the processor couldn’t handle the real-time computations without overheating.
In summary, identifying these integration challenges early—during the design and prototyping phase—can prevent delays, FDA rejections, and even product recalls. Proactive testing and flexible architecture are critical.
🔹 Section 3: Best Practices in Design and Architecture
Key Points:
-
Adopt modular design principles
-
Use standard communication interfaces and APIs
-
Plan for scalability and firmware updates
1. Modular Design Principles
A modular architecture breaks the system into independent, interchangeable components—making development, testing, and updates significantly easier.
-
Benefits of Modular Design:
-
Each module (e.g., heart-rate sensor, display driver, firmware updater) can be developed and tested independently.
-
Changes in one module (like switching to a new sensor model) don’t break the entire system.
-
It speeds up development by allowing parallel work across teams.
-
Example:
In a digital blood pressure monitor, the data acquisition unit, signal processor, and display interface were designed as separate modules. This helped the team identify and fix a bug in the signal processor without affecting the UI.
2. Use Standard Communication Interfaces and APIs
Choosing industry-standard communication protocols and Application Programming Interfaces (APIs) enhances compatibility and long-term maintainability.
-
Common Standards:
-
USB for power/data exchange
-
BLE (Bluetooth Low Energy) for wireless communication
-
HL7 (Health Level Seven) for electronic health data exchange
-
Why It Matters:
Standardization reduces the chance of integration errors and makes it easier to interface with other systems like hospital networks or cloud platforms.
3. Plan for Scalability and Firmware Updates
Devices must adapt over time to include bug fixes, security patches, and new features. Designing for firmware updateability is now essential.
-
Best Practices:
-
Include bootloaders that allow firmware to be updated over-the-air (OTA).
-
Build in sufficient memory to accommodate larger future firmware builds.
-
Use versioning and maintain backward compatibility where possible.
-
Case Insight:
A wearable ECG tracker initially launched with only basic rhythm monitoring. Later, a firmware update added arrhythmia detection—thanks to the device’s scalable and update-friendly architecture.
In essence, good design is not just about making the device work today—it’s about ensuring it can evolve tomorrow. Modular, standards-based, and scalable architecture reduces technical debt and future-proofs medical innovations.
🔹 Section 4: Testing and Validation Strategies
Key Points:
-
Importance of unit, integration, and system-level testing
-
Simulators and emulators for early testing
-
Compliance with regulatory standards (e.g., ISO 13485, IEC 62304)
1. Importance of Unit, Integration, and System-Level Testing
Testing should occur at every stage of development to catch bugs early and ensure the medical device performs reliably.
-
Unit Testing: Verifies individual components (e.g., a function that reads sensor data) work as expected.
-
Integration Testing: Ensures that hardware and software modules communicate correctly.
-
System-Level Testing: Tests the complete device under real-world conditions to validate safety, usability, and performance.
Example:
In a smart insulin pump, unit testing ensured the delivery module dispensed correct doses. Integration testing verified Bluetooth connectivity to a smartphone app. System testing validated the entire workflow, including emergency shutoff in case of user error.
2. Simulators and Emulators for Early Testing
Simulators (software-based) and emulators (hardware-based) allow testing before the final hardware is available.
-
Why Use Them:
-
Reduce time-to-market by enabling parallel hardware and software development.
-
Identify software bugs early, even when the actual hardware is delayed.
-
Simulate edge cases (e.g., sensor failure or extreme inputs) safely.
-
Example:
A company developing a wearable thermometer used a sensor simulator to mimic different temperature inputs. This allowed the firmware team to complete debugging weeks before the actual sensor was integrated.
3. Regulatory Compliance with ISO 13485 and IEC 62304
Medical device software must comply with strict regulatory standards to ensure safety and quality.
-
IEC 62304: Governs software life cycle processes for medical devices.
-
ISO 13485: Specifies quality management systems for design and production.
Best Practices for Compliance:
-
Document every stage of testing and development.
-
Perform risk analysis and mitigation for all software functions.
-
Maintain traceability from requirement to implementation to test.
Tip:
Use automated tools for test management and traceability matrices to stay organized and audit-ready.
Thorough testing isn’t optional—it’s a necessity. It ensures that devices meet both user expectations and regulatory demands, ultimately protecting patient health and the manufacturer’s reputation.
🔹 Section 5: Communication and Collaboration Among Teams
Key Points:
-
Regular interdisciplinary meetings
-
Clear documentation and version control
-
Agile methodologies to manage complexity
1. Regular Interdisciplinary Meetings
Effective integration between hardware and software teams requires ongoing communication.
-
Schedule weekly or bi-weekly stand-ups with cross-functional members (engineers, designers, QA, regulatory experts).
-
Discuss blockers, recent changes, and upcoming tasks.
-
Use shared dashboards (like Jira or Trello) to ensure visibility and alignment.
Example:
In one MedTech project, weekly sync-ups helped engineers quickly identify a mismatch between a sensor’s analog output and the ADC firmware setup. Fixing this early saved weeks of troubleshooting later.
2. Clear Documentation and Version Control
Documentation and source control are vital for avoiding miscommunication and tracking changes.
-
Use tools like Git for source control with proper branching strategies (e.g., GitFlow).
-
Maintain shared documentation using platforms like Confluence, Notion, or Google Docs.
-
Document:
-
Interface definitions (pin maps, baud rates, API endpoints)
-
Firmware update logs
-
System architecture diagrams
-
Pro Tip:
Create a “living document” that evolves with the project. Everyone should have access and contribute to it.
3. Agile Methodologies to Manage Complexity
Agile isn’t just for software—it works wonders in hardware-software co-development too.
-
Break work into small sprints with clear deliverables.
-
Review integration progress regularly and adapt to changes quickly.
-
Prioritize tasks that unblock others, such as hardware specs needed for firmware development.
Hybrid Agile-Waterfall models are also common, especially in regulated environments where documentation and compliance are critical.
Why It Matters:
Without strong collaboration, integration issues get buried until late stages, causing delays and expensive rework. Cross-functional teamwork is the glue that binds successful product development.
🔹 Section 6: Real-World Case Studies and Solutions
Key Points:
-
Case study of a pulse oximeter with firmware errors
-
Solutions applied in wearable cardiac monitors
-
Lessons learned from FDA recall cases
1. Pulse Oximeter Case Study – Firmware Integration Flaws
A leading manufacturer released a pulse oximeter that showed intermittent reading errors.
🔍 Issue Identified:
-
Poor interrupt handling between the optical sensor hardware and the microcontroller’s firmware.
-
The firmware failed to consistently capture photoplethysmography (PPG) signals due to timing mismatches in the interrupt service routine (ISR).
🛠️ Solution Applied:
-
Engineers redesigned the ISR with stricter timing constraints and added buffering for missed interrupts.
-
They also implemented firmware logging, which helped detect race conditions during testing.
📈 Outcome:
-
Post-fix, the error rate dropped by 92%, and device reliability increased significantly.
2. Wearable Cardiac Monitor – Bluetooth Synchronization Issues
A startup building wearable ECG monitors experienced major problems with Bluetooth synchronization, where the data transmission between the device and smartphone app lagged or dropped.
🔍 Issue Identified:
-
The standard BLE protocol introduced latency during data streaming due to overhead and retries.
-
Real-time heart rate analysis was impacted.
🛠️ Solution Applied:
-
The team developed a custom low-latency communication protocol over BLE using direct connection modes and reduced packet size.
-
Software and hardware teams closely collaborated to balance power consumption and maintain signal integrity.
📈 Outcome:
-
Achieved real-time syncing with <100ms delay and increased battery life by 20%.
3. Lessons from FDA Recall Cases
According to the FDA Medical Device Recall Database, a significant number of recalls are due to software-related issues—and many of them are tied to poor integration practices.
🔍 Examples Include:
-
Devices that failed to detect hardware malfunctions because error states weren’t exposed to the software layer.
-
Safety-critical systems that crashed due to mismatched voltage levels not properly handled in firmware.
📢 Key Takeaway:
-
Most failures were preventable with better system-level testing, interdisciplinary collaboration, and early-stage integration validation.
Why These Case Studies Matter:
Real-world failures are powerful reminders that successful hardware-software integration isn’t just about functionality—it’s about safety, compliance, and patient trust. Each mistake is a lesson in the value of planning, testing, and communication.
3. Conclusion
🔄 Summary of Key Points:
Effective hardware-software integration in medical devices is a critical factor in ensuring reliability, safety, and regulatory compliance. Throughout this article, we’ve explored:
-
The fundamentals of integration, emphasizing the need for early cross-disciplinary collaboration.
-
Common challenges, such as communication mismatches, timing issues, and hardware limitations.
-
Best practices in design, including modular architectures, standard interfaces, and scalable firmware.
-
The importance of thorough testing, validation, and regulatory alignment with standards like IEC 62304.
-
The role of team communication and agile workflows in managing complexity.
-
Real-world case studies demonstrating how integration failures can lead to serious device errors—and how they were successfully resolved.
💡 Final Thoughts & Implications:
As medical devices become more intelligent, connected, and wearable, the integration of hardware and software will only become more complex and mission-critical. Whether it’s a smartwatch that monitors cardiac rhythm or an implantable glucose sensor, the expectation for accuracy and safety remains high.
To stay ahead, teams must embrace a structured, proactive approach:
-
Collaborate early and often across disciplines.
-
Plan for scalability, firmware updates, and future innovation.
-
Treat testing as a core development activity, not a final checkpoint.
-
Align development practices with regulatory requirements to reduce recall risks.
Most importantly, recognize that the end-user is often a patient whose life or wellbeing may depend on your device. This makes hardware-software integration not just a technical task—but a moral responsibility.
4. FAQs (Frequently Asked Questions)
1. What are the most common hardware-software integration issues in medical devices?
-
Communication mismatches, such as misconfigured protocols (e.g., UART, SPI, I2C).
-
Timing or synchronization problems, where software and hardware cycles are out of phase.
-
Memory and performance constraints, especially in embedded systems with limited resources.
2. How can early-stage design prevent integration problems?
-
By applying a modular architecture, components can be developed and tested independently.
-
Involving cross-disciplinary teams (hardware, software, systems engineers) early reduces misalignment.
-
Early use of interface specifications and simulators can help validate interactions before the hardware is finalized.
3. What role does regulatory compliance play in integration?
-
Regulatory frameworks like IEC 62304 and ISO 13485 enforce structured design and validation processes.
-
Ensures that safety, performance, and reliability are systematically verified through documentation and testing.
-
Helps avoid costly recalls or rejections from regulatory bodies such as the FDA.
4. Which testing strategies are most effective for embedded medical devices?
-
Unit testing: Validates individual software modules.
-
Integration testing: Ensures communication and functionality between hardware and software.
-
System-level testing: Validates the full device in realistic operating conditions.
-
Simulators and emulators: Useful during early stages to test software without physical hardware.
5. How can teams effectively collaborate during development?
-
Use of Agile methodologies to support iterative testing and continuous integration.
-
Maintain shared documentation platforms (e.g., Confluence, GitHub) to track progress and changes.
-
Schedule regular interdisciplinary meetings for early feedback, faster issue resolution, and better alignment.
5. References
Below is a curated list of credible and authoritative sources that support the strategies and insights discussed in this article. These references are essential for readers seeking to dive deeper into regulatory standards, real-world examples, and best practices in medical device development.
-
IEC 62304 – Medical Device Software – Software Life Cycle Processes
-
International standard defining the requirements for software used in medical devices, including risk management and integration testing.
-
-
ISO 13485 – Quality Management Systems for Medical Devices
-
Provides a framework for ensuring consistent quality in the design, development, and manufacturing of medical devices.
-
-
FDA Medical Device Recall Database
-
Real-world data on past device failures, including recalls due to hardware-software integration issues.
-
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm
-
-
Teixeira, Marie B. (2002). Design Controls for the Medical Device Industry
-
A comprehensive guide to implementing design control processes that ensure compliance and integration success.
-
-
MedTech Dive – News and case studies on medical technology development and innovation.
-
IEEE Access Journal – Technical articles and research papers on medical device systems and integration methods.
File Under: Hardware


Currently it looks like BlogEngine is the best blogging platform available right now. (from what I’ve read) Is that what you are using on your blog?